Regulations
With the aim of harmonizing the Member States laws in relation to the Personal Protection Devices (PPE), on 31 March 2016 the new Regulation 2016/425 / EU on Personal Protective Equipment (PPE) was published in the Official Journal of the European Union. which replaces Directive 89/686/EEC of 21 December 1989.
The regulation applies from April 21st 2018, when Dir 89/686/EEC is repealed. In the new regulation some test procedures have remained unchanged, while others have been modified to provide a better data analysis by the user.
WHAT ARE THE HIGHLIGHTS AND NEWS OF THE REGULATION?
Let’s try to summarize below the news about gloves production:
1) Until the new Regulation 2016/425/EU application, the Dir 89/686/EEC still applies
2) Certifications conforming to Dir 89/686/EEC will be valid until April 21st 2023
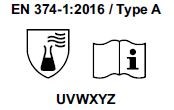
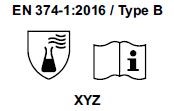
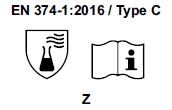
3) Starting from April 21st 2017 the test methods required to obtain the PPE certifications have changed and consequently also the pictograms for EN374 (protective gloves against Chemical Agents and Microorganisms) and for EN388 (protective gloves from risks mechanical)
EN 374 AMENDMENTS
EN ISO 374-1: 2016 Requirements for chemical risks
- The chemical protection index will be expressed only with the pictogram showing the flask. 6 new chemical agents were added to the list of 12 of the old legislation, for a total of 18 chemical agents (chemical agent list). Each chemical agent tested is identified with a letter code, which must be reported under the pictogram.
EN ISO 374-1:2016
| Code letter | Chemical | CAS number | Class |
| A | Methanol | 67-56-1 | Primary alcohol |
| B | Acetone | 67-64-1 | Ketone |
| C | Acetonitrile | 75-05-8 | Nitrile compound |
| D | Dichloromethane | 75-09-2 | Chlorinated paraffin |
| E | Carbon disulphide | 75-15-0 | Organic compound containing sulphur |
| F | Toluene | 108-88-3 | Aromatic hydrocarbon |
| G | Diethylamine | 109-89-7 | Amine |
| H | Tetrahydrofuran | 109-99-9 | Heterocyclic and ether compound |
| I | Ethyl acetate | 141-78-6 | Ester |
| J | n-heptane | 142-82-5 | Saturated hydrocarbon |
| K | 40% Sodium hydroxide | 1310-73-2 | Inorganic base |
| L | 96% Sulphuric acid | 7664-93-9 | Inorganic mineral acid |
| M | 65% nitric acid | 7697-37-2 | Inorganic mineral acid |
| N | 99% acetic acid | 64-19-7 | Organic acid |
| O | 25% ammonium hydroxide | 1336-21-6 | Organic base |
| P | 30% hydrogen peroxide | 7722-84-1 | Peroxide |
| S | 40% hydrofluoric acid | 7664-39-3 | Inorganic mineral acid |
| T | 37% formaldehyde | 50-00-0 | Aldehyde |
Gloves will be classified as:
- TYPE A if they obtain a passage time ³ 30 minutes (level 2) for 6 chemical agents, among those listed in the normative EN 16523-1.
- TYPE B if they obtain a passage time ³ 30 minutes (level 2) for 3 chemical agents, among those listed in the EN 16523-1
- TYPE C if they obtain a passage time ³ 10 minutes (level 1) for only 1 chemical agent, among those listed in the EN 16523-1
EN 374-4:2013 Degradation resistance
The degradation index has been added to the legislation. It’s not is not mandatory to get the certification, but it must be explicitly explained on the user information sheet and also on the packaging of the product. It indicates the percentage of degradation value obtained for each standard, for which resistance to permeation according to EN ISO 374-1: 2016 has been tested.
EN ISO 374-5:2016 Requirements for risks from micro-organisms
EN 374-2: 2003 is replaced by EN ISO 374-5: 2016 and gloves will be classified as:
- PROTECTIVE GLOVES AGAINST BACTERIA AND FUNGI

- PROTECTIVE GLOVES AGAINST BACTERIA AND FUNGI AND VIRUS

Only if the determination of the resistance to the passage of viruses will be performed and passed as required by the ISO 16604: 2004 standard (bacteriophage Phi-X174), the indication “VIRUS” can be applied under the pictogram
NEWS ON EN 388
EN 388 Mechanical risks
For disposable gloves, compliance with EN 388 is no longer required. It has been replaced by the degradation test according to EN 374-4: 2013.
For all the other types of glove, some revisions have been made on the methodologies of CUTTING and ABRASION resistance tests, as per ISO 13997 standard.
- The cut resistance test (Couptest) did not allow to correctly qualify the performance of high shear strength, so it was made more reliable, with a greater control of the blade (blade change every 5 tests).
If the glove specimen does not wear the blade, the Couptest remains the reference test. If the specimen bevels the blade, the reference test must be in accordance with ISO 13997 and a fifth digit should be indicated under the pictogram to indicate the level of shear strength test.
- The abrasion resistance test requires the use of a more consistent quality abrasive paper.
- The possibility of inserting a test (EN 13594: 2015) for impact protection on joints and fingers has been added. The letter “P” is added after the 5-digit pictogram, if the glove passes the test.
![]()
WHAT WILL THE CONSEQUENCES OF THE REGULAR ADJUSTMENT BE?
The Certification Bodies have started to adapt the tests according to the new regulations from April 2017. This means for the economic operators a starting process of products adjustment – products already present or to be introduced on the European market – which will lead to revision of the technical sheets, declarations of conformity and labelling according to the new requirements.
We believe it is plausible, over the next few months, to see changes in certification approaches depending on the response of the tests currently being performed.
Directive 89/686/EEC harmonizes the terms for the marketing and distribution of Personal Protective Equipment within the European Union.
The CE marking shows the compliance with the Directive.
Standard EN 420
The standard specifies the general requirements and tests for the design and manufacturing of the gloves, resistance to water penetration, safety, comfort and efficiency features together with the information concerning the marking and supplementary information provided by the manufacturer applicable to all protection gloves.
EN 388
The EN 388 standard provides the minimum requirements for the mechanical origin risks, due to contact with sharp objects, sharp or protruding simply. The legislation requires four types of test:
A. Abrasion resistance: evaluated as number of cycles required to completely consume the specimen. The results are shown as protection levels 1 to 4.
(Security Level 1: 100 cycles Security Level 2 – 500 cycles; Security level 3 – 2000 cycles; Security Level 4 – 8000 cycles).
B. Shear strength: evaluated as number of cycles required to cut the sample at constant speed. The results are shown as protection levels 1 to 5.
(Security Level 1 – N 1.2 cycles. Security Level 2 – N 2.5 cycles. Security level 3 – N 5 cycles. Security Level 4 – N 10 cycles. Security Level 5 – N 20 cycles).
C. Tear strength: indicates the force required to tear and tear the specimen. The results are shown as protection levels 1 to 4.
(Security Level 1-10 newtons; Protection level 2-25 newtons; Protection level 3-50 newtons; Protection level 4-75 newtons).
D. Resistance to perforation: indicates the force required to be applied to a tip of the standard size to pierce the specimen. The results are shown as protection levels 1 to 4.
(Security Level 1-20 newtons; Protection level 2-60 newtons; Protection Level 3-100 newtons; Protection Level 4-150 newtons).
EN 374
The EN 374 standard specifies the requirements for gloves to protect the user against chemicals and / or microorganisms and is constituted of 3 parts:
EN 374-1: It defines the terminology and general requirements.
EN 374-2: Defines the resistance to penetration of the glove, which means the promotion, at non-molecular level of a chemical substance or a micro-organism through porous material, seams holes or other imperfections in the glove material.
The resistance to penetration of the glove is evaluated with the execution of 2 tests:
–the evidence of air leakage, which involves immersing a glove in water, apply pressure with air inside and check for a leak from any stream of air bubbles on the surface;
–the evidence of loss of water, which provides water to fill a glove and verify the presence of a leak from any of the outside appearance of water droplets.
It is believed that gloves that are resistant to penetration, in accordance with EN 374-2, constitute an effective barrier against microbiological hazards.
The resistance to penetration is also described in ISO 2859 standard, as the acceptable quality level (AQL), which represents the average number of acceptable defective gloves (for presence of holes) present in a production batch.
The lower the AQL, the higher the quality of the glove.
So that they can be certified as medical devices, gloves must have an AQL ≤1,5. The test that determines the AQL of a glove, consists in a test of water-tight, which provides that the gloves are hanging to a filling tube positioned vertically and are filled with about 1000 ml of water at a room temperature. The glove thus filled is visually checked for 2-3 min. and if it does not waste water is considered suitable.
They define 3 levels of AQL:
Level 1: the acceptable quality level (AQL) ≤0,65
Level 2: the acceptable quality level (AQL) ≤1,50
Level 3: the acceptable quality level (AQL) ≤4,00
EN 374 -3: The law defines the resistance of the glove to permeation, which means the promotion at the molecular level of non-gaseous chemicals. Permeation includes three processes: the absorption of the chemical in the contact surface (external) of the glove; the diffusion of the chemical through the glove material; the desorption of the chemical from the opposite (inside) surface of the glove. More simply, the currency permeation for each chemical compound absorption from the outside to the glove, but also the subsequent release from the glove to the hand of the operator.
The resistance of the glove to permeation is evaluated as a measure of the penetration time of the chemical through the glove material under laboratory conditions specifically described in the reference standard.
On the basis of the penetration times obtained, they define 6 performance levels of permeation.
PERFORMANCE REQUIREMENTS PERMEATION
| Measured breakthrough time (minutes) | Permeation level performance |
>10 >30 >60 >120 >240 >480 | 1 2 3 4 5 6 |
The Directive 93/42/EEC on medical devices (MDD 93/42), is a document that sets out the general criteria to be used in the design and construction of certain categories of medical devices.
Standard EN 455 – part 1
The legislation specifies the requirements and provides methods for the testing of medical disposable gloves to determine the absence of holes
- Sampling, control level and AQL:
ERWAN brand examination gloves meet the ISO 2859-1 general inspection level 1 and the level of compliance to the absence of holes, which must be an AQL (Acceptable Quality Level) equal to or less than the world standard for surgical gloves, i.e. 1, 5.
Standard EN 455 – part 2
The EN 455 specifies the requirements and test methods for the physical properties of medical disposable gloves, to ensure an adequate level of protection from mutual contamination of the patient and the user.
- Dimensions:The disposable glove must have a minimum dimension of 240 mm.
- Resistance:Resistance tests are determined “before and after” the aging of the glove, as described in Sections 5.2 and 5.3, in order to ensure the resistance of the glove under various conditions of use.
Standard EN 455 – Part 3
The regulation specifies the requirements relating to the evaluation of biological safety for the medical disposable gloves and clarifies the indications on the labeling and packaging of gloves, and disclosure of information relating to the test methods used.
Standard EN 455 – Part 4
The regulation EN 455-4 indicates the requirements and test methods for establishing the validity period of the product ( “shelf life determination”) and indicate the deadline.
The Regulation 1935/2004/EEC of the European Parliament and of the Council concerning the materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/CE.
All materials and articles intended to come into contact with food shall apply:
- EC Regulation 2023/2006 on good manufacturing practices
- Regulation 1935/2004/EEC to exclude the transfer of substances to foodstuffs in quantities such as to endanger the health or lead to a change in the composition of the food or a deterioration in the organoleptic aspects of the food.
Global Migration tests are performed on the basis of simulants that emulate the behavior of all the edible food.
| Types of food and food simulants by Reg EU 10/2011 and subsequent updates.* | |
| Denominazione | Simulante |
| Food Simulant A | Ethanol 10% (v/v) |
| Food Simulant B | Acetil acid 3% (w/v) |
| Food Simulant C | Ethanol al 20% (v/v) |
| Food Simulant D1 | Etanol al 50% (v/v) |
| Food Simulant D2 | Vegetable oil |
| Food Simulant E | Poly 2,6-diphenyl-p-phenylene oxide (MPPO) |
* Table subject to change for the constant renewal of regulations.
Moreover, some elements are BANNED and kept UNDER CONTROL by constant tests for specific migrations. Some elements must not exceed specific levels (eg phthalates, vinyl chloride, acrylonitrile etc.).
ALL TESTS ARE WE MADE BY EU LABORATORY TESTS and constantly reviewed.
All sectors are constantly evolving and even the examination gloves sector does not escape this law.
In the last two-three years, the disposable gloves consumer is gradually shifting from the use of powdered gloves to the use of powder-free gloves. The reasons for this trend are many, first of all glove’s powder often causes allergic reactions and contamination, which in some areas can be a critical element (mainly in food and healthcare business) and as second reason are today available on the market, at an affordable price, alternative products to powdered gloves.
The potential criticality of the presence of powder in gloves has also been recognized by the American FDA, which in January 2017 issued a decree banning the use in the public health sector of POWDERED GLOVES both surgical and patient examination gloves. The ban concerns only Medical Devices (and does not involve other professional uses) and starts from the concept that the benefits of powdered gloves, mainly related to easier donning are considerably lower than the risks associated with it or the contamination it can cause entering in contact with powder. In the United States, the concept of prevention and precaution prevailed over the negative economic impact of the ban, given the higher cost of powder-free gloves than powdered gloves.
Already in 1997, the US FDA had highlighted the potential risks of powdered medical devices (gloves) in the healthcare industry, but no valid product alternatives were available at that time and therefore no special action was taken.
 AMERICA
AMERICA SCANDINAVIA
SCANDINAVIA SPAIN
SPAIN RUSSIA
RUSSIA AFRICA
AFRICA AUSTRALIA
AUSTRALIA